Packaging Validation
The validation of packaging stems from the need to ensure the quality of products across the many steps in your distribution cycle: from shipping to storage. It is indeed an increasingly common requirement for many product categories, including a strong emphasis on the packaging quality requirements for medical and pharmaceutical industries.


Test for Strength & Integrity
ISTA Packaging Standards
The International Safe Transport Association is an organization dedicated to understanding the expectations of safe transport services. It is the primary source of research protocols for packaging validation, whose methodology has been evolving since 1948.
ASTM Packaging Standards
ASTM is one of the world's leading international quality organisations. The ASTM guidelines are an agreement between consumers, manufacturers, government agencies and scientists from over 140 countries on the implementation of packaging quality and testing requirements.
The complete range of tests
Transport Simulation Testing
- Atmospheric Preconditioning
- Atmospheric Conditioning
- Mechanical Handling Test
- Compression Test
- Concentrated Impact Test
- Free Fall Drop Test
- Fork Handling Test
- Low-Pressure Test
- Loose Load Vibration Test
- Fixed or Random Vibration Test
- Incline Impact Test
- Rotational Drop test
Medistri can validate your packaging according to ISTA 2A, ISTA 3A, ASTM D7386, ASTM D4169.
Should you fully validate your packaging system or should you simply test one particular characteristic of your sterile barrier system, Medistri laboratory is accredited and highly experienced for the most common test method provided in ISO 11607-1.
Sterile Barrier System Integrity Testing
- Accelerated Ageing Tests according to ASTMF1908
- Real-Time Ageing Tests
- Visual Inspection Tests according to ASTMF1886
- Dye Migration Tests according to ASTM F1929 & F3039
- Bubble Emission Tests according to ASTM F2096
- Seal Tensile Strength Test according to ASTM F88
- Peel Strength Test according to EN 868-5
- Burst Test according to ISO2758
For Pharmaceuticals & Medical Devices
Packaging Validation for medical devices & pharmaceutical goods is segmented into three categories.
1. Environmental Conditioning
2. Transport Simulation (also known as Transit Testing)
3. Integrity Testing (also known as Sterile Barrier Integrity Testing)
Packaging Validation allows you to measure the strength of your packaging when it is faced with unknown distribution, logistical & environmental stressors. Once you’ve performed the first two test categories, we finish the packaging validation process with Sterile Barrier Integrity Testing in our laboratory. This last series of tests allows you to ensure that your packaging’s sterile barrier has not been compromised during the previous tests.
According to ISO 11607-1
ISO 11607-1 specifies the requirements related to the compliance of the packaging for sterilised medical devices, including materials, sterile barrier systems and packaging systems.
- Sterile barrier system: “The minimum packaging that prevents the entry of microorganisms and allows the sterile presentation of the product in the place of use”.
- Protective packaging: “A material configuration designed to prevent damage to the sterile barrier system and its contents from the time they are assembled until they are used”.
- Packaging: “A combination of the sterile barrier system and the protective packaging".
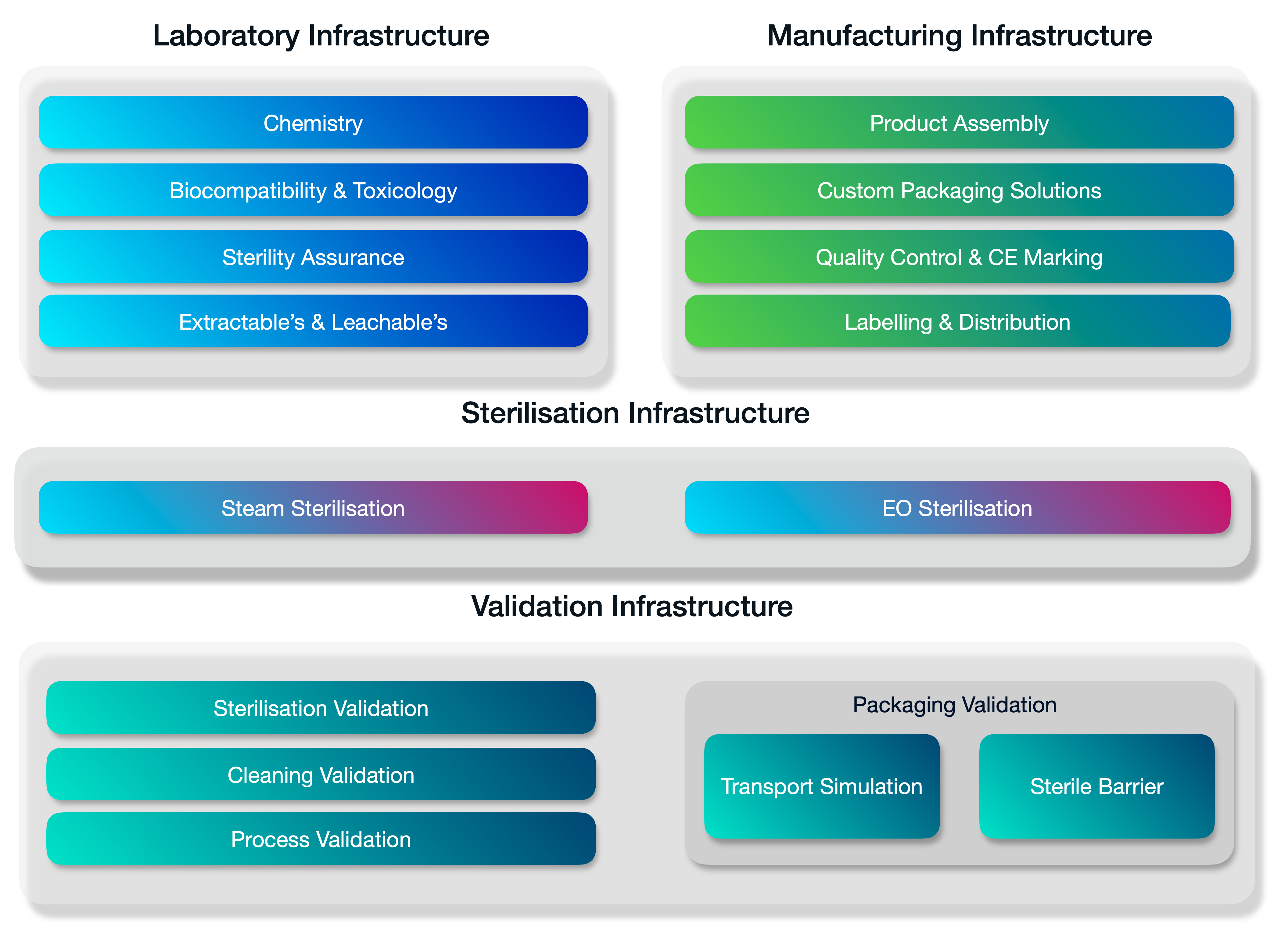
A fully integrated stack of services.
Medistri is continually innovating our in-house range of services. We’re expanding our services to offer a complete integrated suite of solutions for innovative healthcare companies. Organisations of every size — from startups to large enterprises use our suit of services to grow & optimise their business.
Medistri combines all its technical infrastructure together and places quality at the heart of our day-to-day operations. Allowing you to simplify your supply chain management and focus on growth.
Discover how our customers use Medistri's stack of integrated services ➝
Biocompatibility Testing for Medical Devices
Complex Medical Devices need to assess the biocompatibility of their medical device materials and processes by taking a risk-based approach to their biological safety evaluations. Our laboratory takes into consideration the materials, processing, and historical use of the device. This allows to perform a comprehensive assessment of biological responses for each medical device in relation to its safety.
Medistri’s Biocompatibility testing performed according to: ISO 10993
Manufacturing Services
During the development of your product or even at the prototyping phase, Medistri can provide you with a custom solution that fit your manufacturing requirements. From Product Assembly to Integration, Cleaning, Custom Packaging, Labelling, CE Marking, Quality Control and even Distribution & Logistics
Sterilisation Validation
Prior to beginning routine sterilisation, a product with a sterile claim needs to complete a validation process to ensure the Sterility Assurance Level claimed is met.
Work with Medistri to Mitigate risks. Improve your product's safety. Strengthen your Supply Chain.
Why Packaging Validation.
- Shortened packaged development time and confidence in product launch.
- Protection of products and profits with reduced damage and product loss.
- Increase customer satisfaction.
Join a growing number of innovative healthcare companies that are using our services to validate their packaging.
Are you ready to get started?
Contact us and our qualified team will respond.