Contract Sterilisation Services
Engineered for Growth.
Medistri’s sterilisation infrastructure was engineered to allow you to save time and distribute your products on the market faster. We have optimised our sterilisation processes to provide you with the fastest sterilisation services available to date.


Compliance and quality at the heart of our concerns.
Our team includes world-class safety experts, all focused on the different parameters of our cycles to meet your specific needs and the complexity of your sterilised products.
Medistri complies with industry certification standards and has adopted the highest standards of quality assurance.
Sterilisation Technologies
EO Sterilisation
Ethylene Oxide (EO or ETO) sterilisation technology is used for the sterilisation of more than 62% of single use pharmaceutical and medical devices. The process requires the simultaneous control of five variables: product design, gas concentration, temperature, relative humidity, and time of exposure. As a low-temperature sterilisation technology (generally between 40-60°C), it is used particularly for heat-sensible products.
Medistri’s smarter & intelligently designed EO sterilisation cycles can be completed within 4 days from the arrival of your products at our facilities.
Steam sterilisation
Steam sterilisation is a widely used non-toxic sterilisation technology that works by exposing your products to pressurised steam at high temperatures in order to destroy viable microorganisms. Medistri's Steam Sterilisation Infrastructure is fully certified for Medical Device Sterilisation & Pharmaceutical Sterilisation.
Our team focuses on designing custom cycles after studying all the critical parameters of your products to deliver high sterility assurance levels without influencing the pharmaceutical validity of the products.
Sterilisation for Pharmaceutical Vials
A crucial prerequisite of terminal sterilisation is to improve the aseptic manufacturing sterility assurance standard of pharmaceuticals without impacting pharmaceutical validity. Medistri is a global leader in industrial pharmaceutical vial sterilisation. Our infrastructure allows you to sterilise your pharmaceutical vials at industrial scale 24/7 while ensuring the integrity and durability of your products. Medistri is certified GMP compliant by Swissmedic.
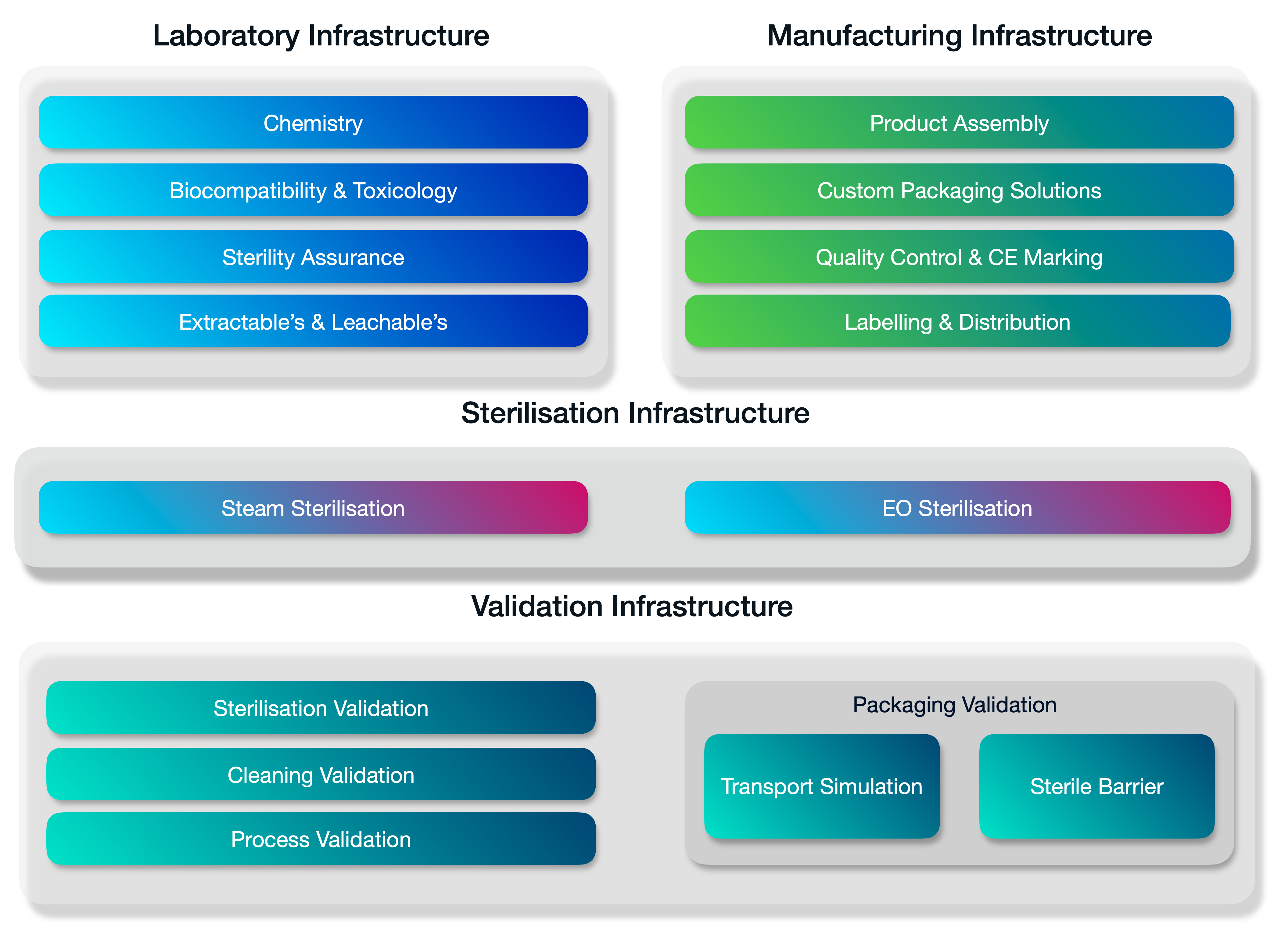
A fully integrated stack of services.
Medistri is continually innovating our in-house range of services. We’re expanding our services to offer a complete integrated suite of solutions for the innovative healthcare companies. Organisations of every size — from startups to large enterprises use our suit of services to grow & optimise their business.
Medistri combines all its technical infrastructure together and places quality at the heart of our day-to-day operations. Allowing you to simplify your supply chain management and focus on growth.
Discover how our customers use Medistri's stack of integrated services
EO Sterilisation
We’ve invested our resources in order to offer a sustainable alternative to the traditional EO sterilisation process. Medistri has introduced a smarter alternative to the market called the “Biological Indicator/ Bioburden Approach”. This approach requires that the Bioburden stays constant over time and less resistant than the biological indicators used.
- Shorter Cycle Duration.
- Reduces Gas Use.
- Sustainable & Environmentally Friendly.
Application Areas
- Pharmaceutical vials.
- Temperature-sensitive products.
- Products with integrated electronics.
- Products with integrated batteries.
- Polymer-based products.
- Implants.
- Surgical Kits.
- Single-use medical devices.
- Drug-Device Combination products.
Key Benefits
- Sterilise Temperature-Sensitive products.
- Sterilise a wide range of products & materials.
- Allows for multiple packaging variations.
- Release your product directly after processing.
- Scalable solution for high growth companies.
- Fast.
Steam sterilisation
Steam sterilisation technology exposes your products with saturated steam under pressure. Steam enhances the ability of heat to kill microorganisms by reducing the time and temperature required to denature or coagulate proteins in the microorganisms. Steam sterilisation cycles generally have three phases including conditioning, exposure and exhaust.
- Between 110-134°C
- Qualified for volumes as large as 400L.
- Fully customisable cycles.
Application area
- Metallic Surgical Instruments.
- Ceramic based products.
- Glass based products.
- Liquids in open or closed containers.
- Gels in open or closed containers.
- Filled Syringes.
- Pharmaceutical Vials.
- Porous Fabrics.
Sterilisation Validation Services
The objective of sterilisation validation is to ensure that the sterilisation method achieves sterility in a replicable manner and that it will not have an adverse effect on the device, packaging or patient health.
Our experts will work closely with your team through the multiple phases of the process to create a sterilisation validation report. The validation report details the process analysis and test results for your validation process. This ensures transparency and traceability at every stage of the project. Our experts gather all the data, present the results and make recommendations in a transparent, detailed and accurate manner.
Medistri's accredited laboratory performs all the necessary tests in-house to develop new test specifications that match the complexity of your products.
- Ethylene oxide validations are performed in accordance with ISO11135.
- Steam sterilisation validations are performed in accordance with ISO17665.
Are you ready to get started?
Contact us and our qualified team will respond.